Oracle IAS, the best coaching institute for UPSC/IAS/PCS preparation in Dehradun brings to you UKPCS Science Chemistry (paper 6).
Sodium carbonate (also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate), Na2CO3, is the water-soluble sodium salt of carbonic acid.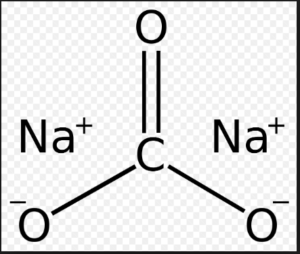
Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber (used to create potash), they became known as “soda ash”. It is synthetically produced in large quantities from salt (sodium chloride) and limestone by a method known as the Solvay process.
Uses:
- By far the largest consumption of sodium carbonate is in the manufacture of glass, paper, rayon, soaps, and detergents. It is also used as a water softener, since carbonate can precipitate the calcium and magnesium ions present in “hard” water.
- Sodium carbonate is a food additive (E500) used as an acidity regulator, anticaking agent, raising agent, and stabilizer.
- Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Production:
- Mining: TRONA is the ore from where it is extracted.
- Several “halophyte” (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century.
- LEBLANC PROCESS:
In 1791, the French chemist Nicolas Leblanc patented a process for producing sodium carbonate from salt, sulfuric acid, limestone, and coal. First, sea salt (sodium chloride) was boiled in sulfuric acid to yield sodium sulfate and hydrogen chloride gas, according to the chemical equation
2 NaCl + H2SO4 → Na2SO4 + 2 HCl
Next, the sodium sulfate was blended with crushed limestone (calcium carbonate) and coal, and the mixture was burnt, producing calcium sulfide.
Na2SO4 + CaCO3 + 2 C → Na2CO3 + 2 CO2 + CaS
- SOLVAY PROCESS: In 1861, the Belgian industrial chemist Ernest Solvay developed a method to convert sodium chloride to sodium carbonate using ammonia.
- Contact us for:-
- IAS coaching in Dehradun (Uttarakhand)
- UKPCS/UPPCS/UPPSC Mains coaching in Dehradun (Uttarakhand)
- Current Affairs classes in Dehradun (Uttarakhand)
- For getting detailed feedback on your answers and improve answer writing
- Phone Number:–9997453844
- Contact us for:-
- UKPCS Mains Mock Test Series: Lower + Upper Combo - July 2, 2025
- UKPCS 2026 Complete Prelims + Mains Course - June 21, 2025
- UKPCS Mains Lower Test Series 2025 - June 19, 2025
