Oracle IAS, the best coaching institute for UPSC/IAS/PCS preparation in Dehradun brings to you UKPCS Science Chemistry (paper 6).
Petroleum products: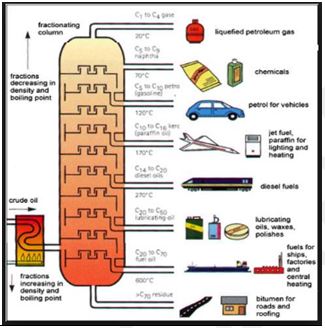
Fractional distillation of crude petroleum and products
Gases
Gaseous refinery products include hydrogen, fuel gas, ethane, propane, and butane. Most of the hydrogen is consumed in refinery desulfurization facilities, which remove hydrogen sulfide from the gas stream and then separate that compound into elemental hydrogen and sulfur; small quantities of the hydrogen may be delivered to the refinery fuel system. Refinery fuel gas varies in composition but usually contains a significant amount of methane; it has a heating value similar to natural gas and is consumed in plant operations. Periodic variability in heating value makes it unsuitable for delivery to consumer gas systems. Ethane may be recovered from the refinery fuel system for use as a petrochemical feedstock. Propane and butane are sold as liquefied petroleum gas (LPG), which is a convenient portable fuel for domestic heating and cooking or for light industrial use.
Gasoline
Motor gasoline, or petrol, must meet three primary requirements. It must provide an even combustion pattern, start easily in cold weather, and meet prevailing environmental requirements.
Octane rating
In order to meet the first requirement, gasoline must burn smoothly in the engine without premature detonation, or knocking. Severe knocking can dissipate power output and even cause damage to the engine. When gasoline engines became more powerful in the 1920s, it was discovered that some fuels knocked more readily than others. Experimental studies led to the determination that, of the standard fuels available at the time, the most extreme knock was produced by a fuel composed of pure normal heptane, while the least knock was produced by pure isooctane. This discovery led to the development of the octane scale for defining gasoline quality. Thus, when a motor gasoline gives the same performance in a standard knock engine as a mixture of 90 percent isooctane and 10 percent normal heptane, it is given an octane rating of 90.
There are two methods for carrying out the knock engine test. Research octane is measured under mild conditions of temperature and engine speed (49 °C [120 °F] and 600 revolutions per minute, or RPM), while motor octane is measured under more severe conditions (149 °C [300 °F] and 900 RPM). For many years the research octane number was found to be the more accurate measure of engine performance and was usually quoted alone. Since the advent of unleaded fuels in the mid-1970s, however, motor octane measurements have frequently been found to limit actual engine performance. As a result a new measurement, road octane number, which is a simple average of the research and motor values, is most frequently used to define fuel quality for the consumer. Automotive gasolines generally range from research octane number 87 to 100, while gasoline for piston-engine aircraft ranges from research octane number 115 to 130.
Each naphtha component that is blended into gasoline is tested separately for its octane rating. Reformate, alkylate, polymer, and cracked naphtha, as well as butane, all rank high (90 or higher) on this scale, while straight-run naphtha may rank at 70 or less. In the 1920s it was discovered that the addition of tetraethyl lead would substantially enhance the octane rating of various naphthas. Each naphtha component was found to have a unique response to lead additives, some combinations being found to be synergistic and others antagonistic. This gave rise to very sophisticated techniques for designing the optimal blends of available components into desired grades of gasoline.
The advent of leaded, or ethyl, gasoline led to the manufacture of high-octane fuels and became universally employed throughout the world after World War II. However, beginning in 1975, environmental legislation began to restrict the use of lead additives in automotive gasoline. It is now banned in the United States, the European Union, and many countries around the world. The required use of lead-free gasoline has placed a premium on the construction of new catalytic reformers and alkylation units for increasing yields of high-octane gasoline ingredients and on the exclusion of low-octane naphthas from the gasoline blend.
High-volatile and low-volatile components
The second major criterion for gasoline—that the fuel be sufficiently volatile to enable the car engine to start quickly in cold weather—is accomplished by the addition of butane, a very low-boiling paraffin, to the gasoline blend. Fortunately, butane is also a high-octane component with little alternate economic use, so its application has historically been maximized in gasoline. Another requirement, that a quality gasoline have a high energy content, has traditionally been satisfied by including higher-boiling components in the blend. However, both of these practices are now called into question on environmental grounds. The same high volatility that provides good starting characteristics in cold weather can lead to high evaporative losses of gasoline during refueling operations, and the inclusion of high-boiling components to increase the energy content of the gasoline can also increase the emission of unburned hydrocarbons from engines on start-up. As a result, since the 1990 amendments of the U.S. Clean Air Act, much of the gasoline consumed in urban areas of the United States has been reformulated to meet stringent new environmental standards. At first these changes required that gasoline contain certain percentages of oxygen in order to aid in fuel combustion and reduce the emission of carbon monoxide and nitrogen oxides. Refiners met this obligation by including some oxygenated compounds such as ethyl alcohol or methyl tertiary butyl ether (MTBE) in their blends. However, MTBE was soon judged to be a hazardous pollutant of groundwater in some cases where reformulated gasoline leaked from transmission pipelines or underground storage tanks, and it was banned in several parts of the country. In 2005 the requirements for specific oxygen levels were removed from gasoline regulations, and MTBE ceased to be used in reformulated gasoline. Many blends in the United States contain significant amounts of ethyl alcohol in order to meet emissions requirements, and MTBE is still added to gasoline in other parts of the world.
Value Addition UKPCS Mains : Uttarakhand Special (Click Here)
UKPCS Mains Study Material subject wise
The notes are strictly as per UKPCS syllabus (topic wise):
Individual Polity Cost: Rs. 1500/- (including shipping)
Individual S&T Cost: Rs. 1500/- (including shipping)
Individual Geography Cost: Rs. 1500/- (including shipping)
Individual Economics Cost: Rs. 1000/- (including shipping)
Individual Ethics Cost: Rs. 1000/- (including shipping)
Individual History Cost: Rs. 1500/- (including shipping)
- UKPSC प्रारंभिक परीक्षा स्टडी मटेरियल 2026 (Upper & Lower) | Oracle IAS - January 3, 2026
- UKPCS 2026 प्री परीक्षा कोर्स : छात्रवृत्ति टेस्ट: Oracle IAS - December 30, 2025
- UKPSC 2026 के लिए उत्तराखंड प्रारंभिक परीक्षा कोर्स 2026 || Oracle IAS - December 15, 2025
