Oracle IAS, the best coaching institute for UPSC/IAS/PCS preparation in Dehradun brings to you UKPCS Science Chemistry (paper 6)- Methanol
ALCOHOL
In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol (ethyl alcohol), the predominant alcohol in alcoholic beverages. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.
Alcohol has a long history of several uses worldwide. It is found in alcoholic beverages sold to adults, as fuel, and also has many scientific (anti-freeze, preservative, solvent), medical (antiseptic), and industrial uses.
Methanol, also known as methyl alcohol among others, is a chemical with the formula CH3OH (often abbreviated MeOH). It acquired the name “wood alcohol” because it was once produced chiefly as a byproduct of the destructive distillation of wood. Today, industrial methanol is produced in a catalytic process directly from carbon monoxide, carbon dioxide, and hydrogen.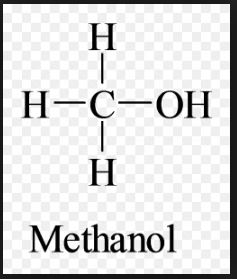
It is the simplest alcohol, being only a methyl group linked to a hydroxyl group. It is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to that of ethanol (drinking alcohol). However, unlike ethanol, methanol is highly toxic and unfit for consumption.
Methanol when drunk is metabolized first to formaldehyde and then to formic acid or formate salts. These are poisonous to the central nervous system and may result in blindness, coma, and death. Because of these toxic properties, methanol is frequently used as a denaturant additive for ethanol manufactured for industrial uses. This addition of methanol exempts industrial ethanol (commonly known as “denatured alcohol” or “methylated spirit”) from liquor excise taxation in the US and some other countries.
Production
From synthesis gas
Carbon monoxide and hydrogen react over a catalyst to produce methanol. Today, the most widely used catalyst is a mixture of copper and zinc oxides, supported on alumina.
CO + 2 H2 → CH3OH
Other
The catalytic conversion of methane to methanol has long been sought as a route to methanol. This route is effected by enzymes such as methane mono-oxygenases but commercial routes remain elusive because of the tendency for over-oxidation, i.e., methanol is more readily oxidized than methane.
UKPCS Mains Study Material subject wise
The notes are strictly as per UKPCS syllabus (topic wise):
Individual Polity Cost: Rs. 1500/- (including shipping)
Individual S&T Cost: Rs. 1500/- (including shipping)
Individual Geography Cost: Rs. 1500/- (including shipping)
Individual Economics Cost: Rs. 1000/- (including shipping)
Individual Ethics Cost: Rs. 1000/- (including shipping)
Individual History Cost: Rs. 1500/- (including shipping)
- UKPSC प्रारंभिक परीक्षा स्टडी मटेरियल 2026 (Upper & Lower) | Oracle IAS - January 3, 2026
- UKPCS 2026 प्री परीक्षा कोर्स : छात्रवृत्ति टेस्ट: Oracle IAS - December 30, 2025
- UKPSC 2026 के लिए उत्तराखंड प्रारंभिक परीक्षा कोर्स 2026 || Oracle IAS - December 15, 2025
